BioBridge
Engineered for Patient Care

The BioBridge® Collagen Matrix is a CE mark Class III device approved for use in the surgical treatment of lymphedema.
This novel thread-like surgical mesh is made of medical-grade collagen and fabricated using Fibralign’s patented Nanoweave® technology to provide the mechanical properties needed to support the body’s own repair process in restoring lymphatic function. BioBridge is implanted using minimally invasive surgical techniques and designed to safely and fully resorb after it completes its function.
BioBridge is the result of an intensive multi-year research effort in collaboration with Stanford University and the University of California, San Francisco (UCSF) to optimize the scaffold’s nanostructure and key parameters to support lymphatic repair.
While BioBridge is a new and much needed breakthrough treatment option, it has already been used in over 60 surgical cases where it has proven to be safe and effective in addressing both upper and lower limb lymphedema. These case studies have shown significant reduction in limb volume, with many patients returning to within normal range.

Case Studies
Example case studies* with “Before” and “After” images of upper and lower limb lymphedema after surgical treatment with BioBridge in support of lymph node transfer (LNT):
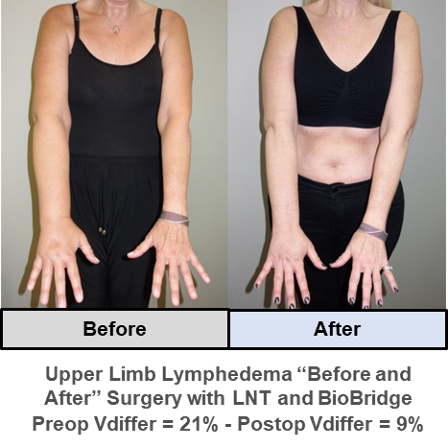
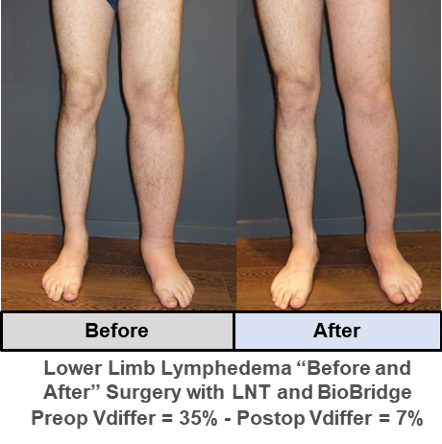

* Provided courtesy of Dr. Dimitris Dionyssiou, THE Lymphedema Clinic (Thessaloniki, Greece)
Proven Performance and Safety
BioBridge Up Close


- Bioengineered native tissue structure produced from only highly purified Type 1 porcine atelocollagen (pepsin treated)
- Biocompatible, minimizes patient immunogenic response
- Design to integrate into tissue and safely fully resorb
- Highly porous multilumenal structure, promotes capillary flow
- Ready to use, provided in single-use sterile packaging (e-beam)
Indications
The BioBridge® Collagen Matrix is intended to reinforce soft tissue where weakness and deficiencies exist, specifically lymphatic tissue repair after or in conjunction with surgical procedures used to address lymphedema (lymph node transfer, lymphaticovenous anastomosis).
BioBridge is not intended to replace normal body structure or provide the full mechanical strength to support soft tissue repair.
Contraindications
BioBridge is contraindicated for use in any patient with known sensitivity to porcine products.
Use of this product in applications other than those indicated has the potential for serious complications, such as suture pullout or failure of the repair.
Proper surgical procedures and techniques are the responsibility of the medical professional. Each surgeon must evaluate the appropriateness of the procedure used based on the medical training and experience, along with the specific patient condition. Strict aseptic techniques should be followed.
Rx Only. For safe and proper use of this device, refer to Instructions For Use (IFU).
BioBridge Up Close


For more information
BioBridge Surgical Usage
Video presentations and tutorials demonstrating different use cases and techniques for implanting the BioBridge® in the treatment of lymphedema.
Animations developed by Dr. Dimitris Dionysiou, THE Lymphedema Clinic.
More to come!
Dr. Dung Nguyen, Stanford Medicine presentation at the recent online conference Evolution in Lymphedema Microsurgery presented by the Hellenic Society for Reconstructive Microsurgery (HSRM) – December 04, 2020
“Lymphatic Microsurgery and Lymphagiogenesis Reinforcement with Nanofibrillar Collagen Scaffolds Implantation”
Animation of Vascularized Lymph Node Transfer (VLNT) with BioBridge for Treatment of Upper Limb Lymphedema
Animation of VLNT with BioBridge for Treatment of Lower Limb Lymphedema
Animation of “Local Flap”, a new surgical technique using BioBridge involving moving a local flap containing a lymph node into the treatment area.
(Technique developed by Dr. Dimitris Dionysiou)
